Complications from dual roles of sodium hydride as a base and as a reducing agent. | Semantic Scholar
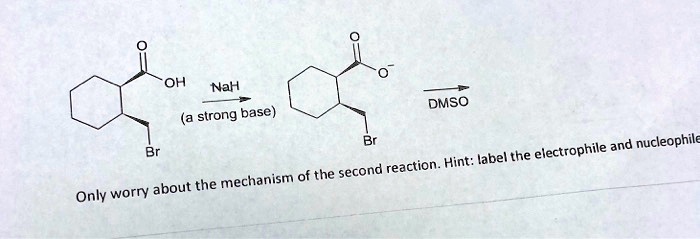
SOLVED: OH NaH DMSO strong base) and nucleophile Hint: label the electrophile second reaction mechanism of the Only worry about the

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

The hydride ion H^(ɵ) is a stronger base than hydroxide ion. Which of the following reaction would occur if NaH is dissolved in water