Welcome to Chem Zipper.com......: 1 mole of N2 and 3 moles of H2 are mixed in 8.21 lit. container at 300 K to form NH3 . If at equilibrium, average molecular mass

Calculate molecular weight Nitrogen|Molar mass of N2|Molecular weight Nitrogen |Nitrogen Molar mass - YouTube
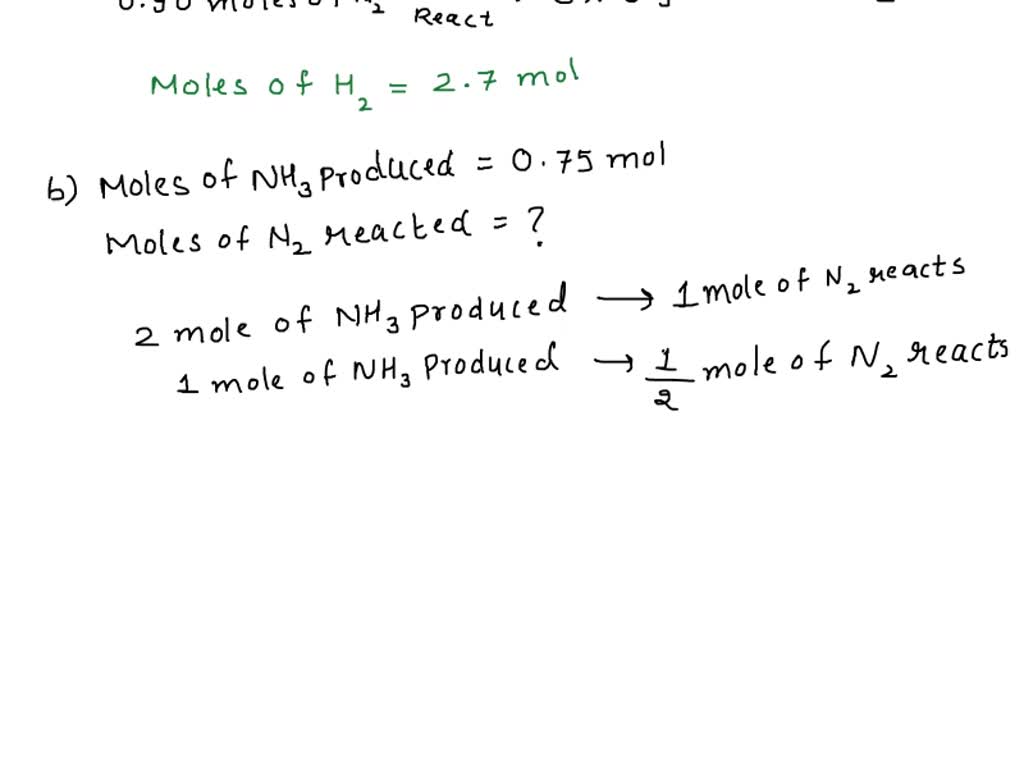
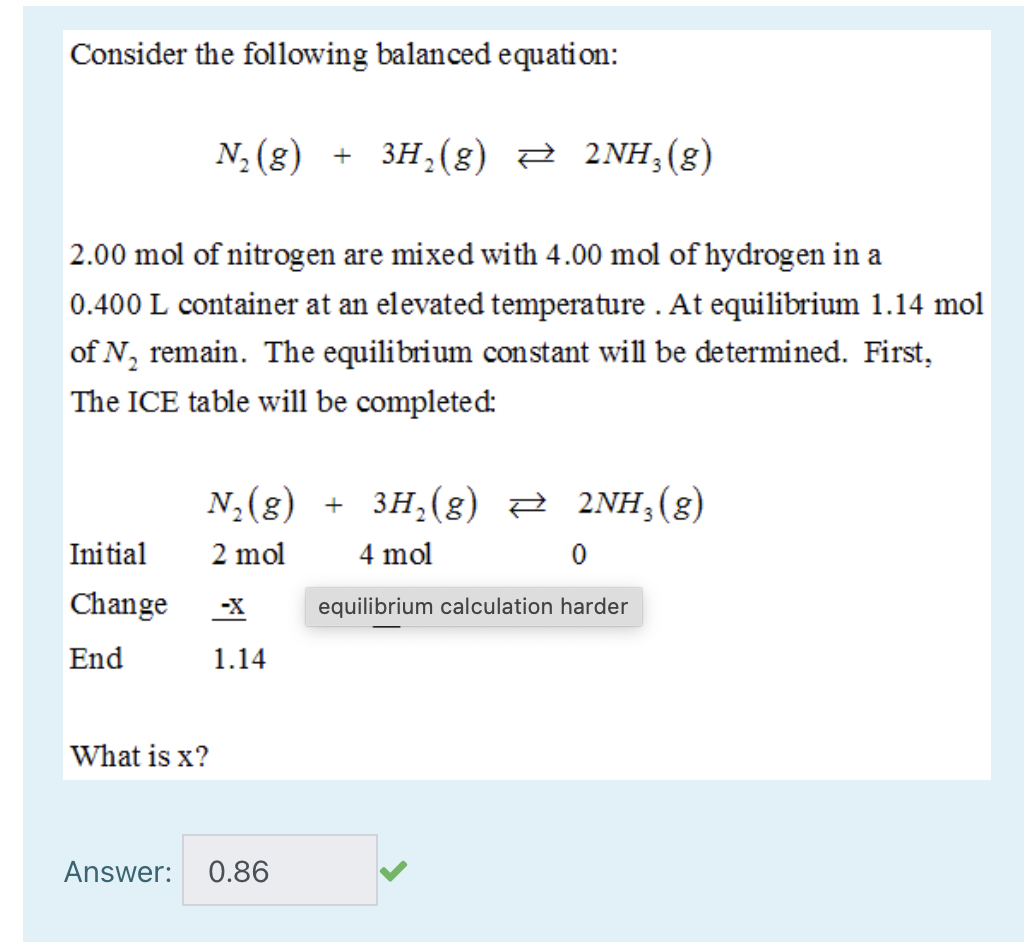
SOLVED: Ammonia is produced by the reaction of hydrogen and nitrogen as follows: N2(g)+3H2(g)→2NH3(g)ammonia A) How many moles of H2 are needed to react with 0.90 mol of N2? Express the number

SOLVED: In the reaction of nitrogen gas, N2, with hydrogen gas, H2, to form ammonia gas, NH3, how many moles of hydrogen are needed to react with 1.22 mol of nitrogen?
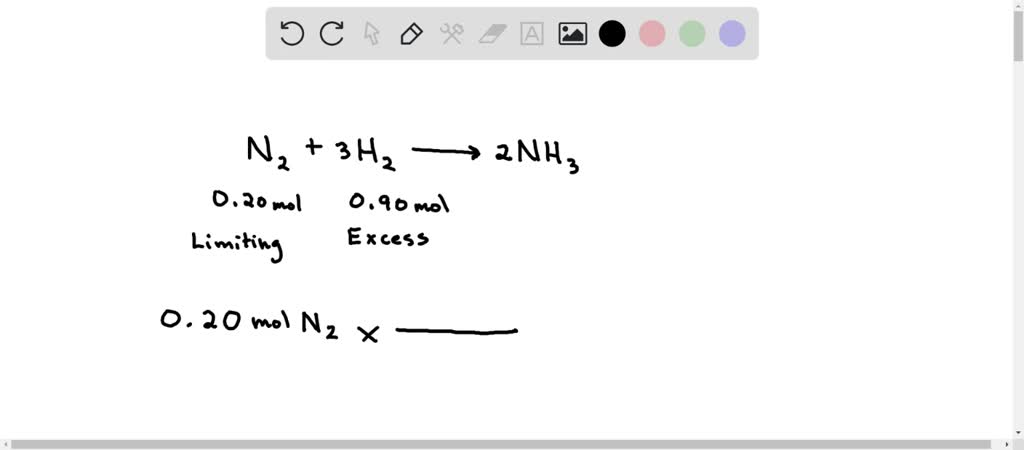
SOLVED: The reaction below is 0.20 mol of N2 and 0.90 mol of H2. How many moles of H2 gas will be left unreacted after the reaction has gone to completion? N2 (

Calculate the volume occupied by 4 mole of an ideal gas at 2.5 × 10^5Nm^-2 pressure and 300 K temperature.